“Nano-Taxis” Shuttle Therapeutics to Neurons
Technion scientist creates innovative “nano-taxis,” capable of shuttling therapeutics directly to neurons, paving the way for treatment of neurodegenerative diseases and traumatic brain injuries
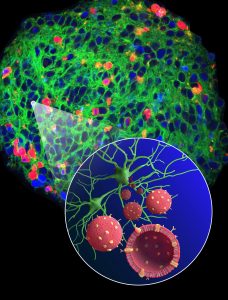
Technion – Israel Institute of Technology Assistant Professor Assaf Zinger and Dr. Caroline Cvetkovic from the Center for Neuroregeneration at the Houston Methodist Research Institute have created a novel means of delivering medicine to neurons in a targeted manner. They developed biomimetic nano-vesicles, or nature-mimicking, nanometer-scale “vehicles,” capable of specifically targeting neurons (i.e., nerve) cells. This tool paves the way for the treatment of multiple neurodegenerative diseases and traumatic brain injuries.
Their findings were recently published in Advanced Science.
Drug delivery is a major challenge that must be overcome in drug development, and it is one of the focus areas of the Wolfson Faculty of Chemical Engineering at the Technion. It is not enough that a substance can lead to the desired therapeutic effect in a specific cell. This therapeutic substance must also reach these cells without being changed or destroyed en route, and it must not end up in other organs if it might cause harm there.
In explaining what led him to this study, Dr. Zinger said, “One Saturday, my family and I were dining with friends. Their little girl has a neurodegenerative disorder; she can’t speak and has a motor disorder. I wanted to help her.”
Dr. Zinger was already working on various types of biomimetic nano-vesicles. These nano-vesicles are similar in their basic structure to human cells but much smaller – one millionth of a hair’s width in diameter. They can carry within them cargo that needs to be delivered to the cells – medication, mRNA, etc.
The targeting of these nano-vesicles is achieved by incorporating specific cell membrane-derived proteins on their surface, thus letting them be recognized and taken in by the correct cells. These specific proteins that cover the surface of these nano-vesicles are naturally used by the body to identify its cells and this is what biomimicry is all about. In essence, the nano vesicles (or taxis) masquerade as neurons, resulting in their being recognized and welcomed by other neurons, thereby making it possible for them to deliver their therapeutic cargo.
Potentially revolutionizing the treatment of neurodegenerative disorders and traumatic brain injuries
These findings have broad implications. More than one neurodegenerative disorder might be treated if the correct medicine or genetic cargo (e.g., mRNA, SiRNA, miRNA) could be delivered to the brain. But these are not the only possible applications.
“With this, we can also potentially revolutionize the treatment of traumatic brain injuries,” Prof. Zinger explained. “In the case of a car accident and or a sports injury, as examples, the brain is first damaged by the impact, as it is struck against the skull. As a result, multiple brain cells are damaged. This starts a process of inflammation. If we could immediately deliver anti-inflammatory drugs to the brain, we could reduce the inflammatory processes, and hopefully prevent fatalities and long-term disabilities.”
The lion’s share of this study was conducted by Dr. Zinger at the Houston Methodist Research Institute and Houston Methodist Hospital as part of his postdoctoral fellowship. Dr. Zinger recently opened a multidisciplinary laboratory at the Technion, in the Wolfson Faculty of Chemical Engineering. His lab aims to create advanced bioinspired technologies and translational therapeutics through a highly multidisciplinary approach. Specifically, Dr. Zinger’s group will integrate in vitro and in vivo models with imaging, molecular biology, and chemical techniques to design novel nano-based technologies that will achieve organ- and cell-specific targeting for improved therapeutic outcomes in different brain and neural diseases, injuries, and various cancers.
To read the full article, click here.