Diverse Utility Found in Antimicrobial Peptide’s Fibril Structure
The elucidation of the unique atomic structure of a human-derived antibacterial peptide fiber has applications in nanotechnology, regenerative medicine, bioengineering, and more.
Antimicrobial peptides (AMPs) are an important part of the immune system and can self-assemble, often enhancing their antimicrobial activity. Professor Meytal Landau and Ph.D. student Yizhaq Engelberg, both of the Technion Faculty of Biology, have uncovered the atomic structure that gives a human-derived AMP the advantageous ability to form active and highly stable materials. Their discovery, published in the journal Nature Communications, has the potential to enable the design of similar artificial materials for technological and medical applications such as treating infections and even killing cancer cells.
The study focused on elucidating the structure of the active core of the human AMP: LL-37 (residues 17-29). The researchers found that LL-37’s core is comprised of a self-assembled, ribbon-like protein fiber of densely packed helices. The fiber’s surface properties can destroy various microbes, such as bacteria, by disrupting the outer membrane of the microbe. Further experimental observation demonstrated that the unique structure is chemically stable and possesses high heat resistance.
In addition to being used as an antibacterial therapy – which is very relevant in today’s age of antibiotic resistance – the researchers envision that the fiber’s nanostructure can be used to create scaffolds for long-lasting and durable biomaterials. The finding opens the door to applications in nanotechnology, regenerative medicine, and bioengineering – such as an antimicrobial coating on medical devices.
The mapping of the peptide structure was conducted and based on research done at the European Synchrotron Radiation Facility (ESRF), in Grenoble, France; at the PETRA III storage ring; at DESY particle accelerators in Hamburg, Germany and; at the Technion: Center for Structural Biology (TCSB), Russell Berrie Electron Microscopy Center of Soft Matter, and the Life Science and Engineering Infrastructure Center.
The researchers’ discovery was lauded by The Israel Society for Microscopy (ISM). The Society recently announced it will award the 2020 Lev Margulis Memorial Prize to Yizhaq Engelberg, for this research breakthrough. The award committee noted that the discovery is an “impressive achievement in the study of the structure of a human antimicrobial peptide” and that it is expected to lead to diverse applications in biotechnology, nanotechnology, antibacterial drug production, tissue restoration, and more.
The study was supported by the Israel Science Foundation (ISF); the Israel Ministry of Science, Technology, and Space; the iNEXT consortium of Instruct-ERIC; and the US-Israel Binational Science Foundation (BSF).
For the article in Nature Communications click here
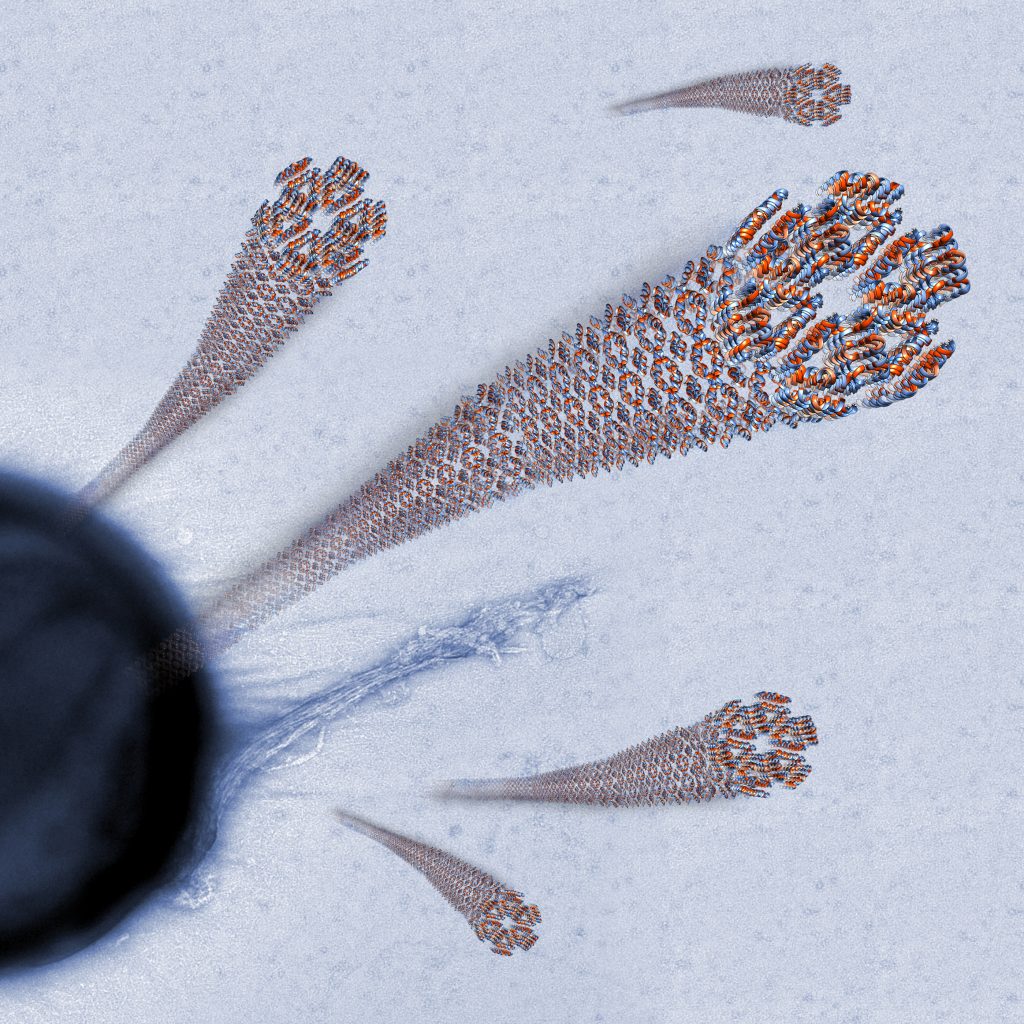
The fiber structure of antimicrobial peptide LL-37 (17-29) uncovered in the present study interacting with bacterial cells. (Credit: Sharon Amlani )




