‘Meta-Synergy’ for the Battle Against Cancer
Technion lab develops an innovative tool for effectively combining cancer drugs, in a powerful synergy of biology, chemistry, and artificial intelligence
Professor Yosi Shamay and his group at the Technion – Israel Institute of Technology’s Faculty of Biomedical Engineering have made major headway in a remarkable new approach to cancer treatment. Based on a new concept called “meta-synergy,” the group developed a powerful artificial intelligence (AI) tool that makes it possible to find drug combinations that are significantly more powerful than each drug individually.
Cancer treatments often employ combination therapy, where different drugs work together in synergy to combat a tumor more effectively than they would do individually. This approach may also prevent the tumor from developing resistance to treatment.
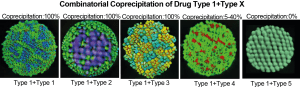
Prof. Shamay’s group has taken this idea to the next level by identifying pairs of drugs that do more than work together biologically to attack the tumor. They also chemically assemble into combined nanoparticles. The researchers describe this synergy of synergies as “meta-synergy,” a cooperative interaction that produces a greater combined effect beyond standard synergy. The resulting nanomedicines are particularly effective at targeting cancer cells and highly successful in fighting tumors, while being less toxic to the patient and causing fewer side effects.
The AI system developed by the team uses text mining to gather information about biological synergy from published articles, compiling the pairs it finds into a comprehensive database. It then predicts which drug duos can chemically self-assemble or join together to form nanoparticles.
Like a talented matchmaker, this AI model pairs drugs based on their biological compatibility and their potential to create nanoparticles together, leading to effective “meta-synergistic” drug pairs. It also feeds an online tool that identifies the most promising drug pairs for different types of cancer. It has proposed 1,985 drug combinations for synergistic nanomedicines for 70 types of cancer.
One example of the model’s abilities is a highly effective drug pair for treating head and neck cancer that it discovered. The two drugs, Bortezomib and Cabozantinib, are already approved for cancer treatment – the first for blood cancers, and the second for liver, kidney, and thyroid cancers. The combination of the two was proven effective and produced fewer side effects than using either of the drugs individually.
“The development of meta-synergy on the nanometric level is a very complex challenge,” Prof. Shamay explained. “It necessitates the introduction of (at least) two drugs simultaneously into the same delivery system that would lead them to the desired destination in the body. Our research has shown, both in a computational demonstration (cheminformatics and artificial intelligence) and in live experiments, that the combination we proposed indeed leads the drugs to the tumor and releases them there — and that this therapy is very effective in treating the disease. Beyond the specific combination we demonstrated in our paper, we believe that the concept of meta-synergy will lead to additional breakthroughs in the fight against cancer.”
The study was conducted at the Shamay Lab for Cancer Nanomedicine and Nanoinformatics. It was led by Ph.D. student Dana Meron Azagury, whose focus was on the biology and chemistry side of the research, and M.Sc. student Ben Friedmann, whose developed the AI model. Combining the two disciplines in perfect synergy could enable the lab to break new ground and benefit cancer patients.
The study was published in the Journal of Controlled Release.
The research was supported by the National Science Foundation (ISF).
For the article in the Journal of Controlled Release, click here.