Paving the way for Cancer Vaccines
Technion and Sheba scientists develop a novel technique, improving diagnosis and immunotherapy treatment for lung cancer
“It is basically a ‘therapeutic cancer vaccine’,” Prof. Arie Admon from the Technion Faculty of Biology explains the mechanism behind the immunotherapy cancer treatment that is the focus of his lab. “It activates the body’s own immune system against the tumor.” Immunotherapy treatments are coming to prominence in recent years, surpassing traditional chemotherapy in its ability to treat more than one type of cancer. Prof. Admon aims to increase the scope of cancers that can be treated by immunotherapy, and to reduce the treatment’s cost. The work of his Ph.D. student Sofia Khazan-Kost, recently published in Journal for Immunotherapy in Cancer (JITC) does just that.
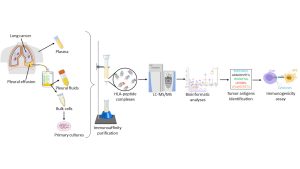
Khazan-Kost’s study, an initiative of Dr. Michael Peled, senior physician at Sheba Medical Center, focused on lung cancer patients. Lung cancer is the most common cause of cancer-related death in both men and women, killing over 1.5 million people around the world annually. Khazan-Kost developed and proved the effectiveness of a simple technique to collect patient-specific tumor peptides that would improve matching the precise treatment to each individual patient. The peptides, which are short bits of protein, were collected from the pleural effusion of lung cancer patients – from liquid discharge that amasses in the patients’ chest and needs to be routinely removed, as it restricts breathing. This liquid would normally be disposed of. Khazan-Kost’s technique, therefore, doesn’t subject the patient to any additional tests, but makes use of “trash”.
Our cells routinely present on their surface, with the help of molecules called HLA, bits and pieces of the proteins (peptides) degraded inside. It is one of the ways each cell communicates to its environment what goes on inside it. This communication becomes important when the cell is infected by a virus, in which case it will display small pieces of viral proteins; or, if the cell turns cancerous, in which case various peptides from aberrant proteins will be displayed. The immune system, seeing these, is supposed to attack and destroy the cell, protecting the body from the disease.
A vaccine, such as the Coronavirus vaccine we’ve become familiar with, induces the immune system to peptides from the bacteria or virus it’s supposed to protect against, in this way “training” it and improving its response. A cancer vaccine is intended to work similarly, training the immune system to attack cancer cells. However, unlike bacteria and viruses, cancer is not an intruder in the body that can be easily “shown” to the immune system. It is an abnormality that occurs within the cells of the body. The aberrant peptides that the diseased cell presents can thus be as different between patients as one patient is from another. This makes generating a vaccine to help multiple patients an extremely complicated task.
The key to Khazan-Kost’s technique are the HLA molecules that present the aforementioned peptides on the cell surface. These molecules, have some variation between people, and are in fact key to transplant organ matching. They are located in the outer membrane of almost every cell in the body, and are also, together with the peptide cargo they carry, released by the cells into the different body fluids, such as blood and the lymph. In advanced stage lung cancer patients, they also find their way into the pleural effusion. Researchers have tried to use HLA molecules isolated from the cancer cells to acquire tumor-specific peptides for immunotherapy development. However, the quantity of such molecules in a tumor needle biopsy was found to be insufficient. Pleural effusions, which need to be removed from the patient’s lungs as part of treatment, provided the needed access point.
Prof. Admon’s team were able to filter out a large amount of HLA molecules from patients’ pleural effusions, and then used mass spectrometry analysis to identify the peptides that the HLA molecules presented. Using this information, they were able to reconstruct the aberrant tumor-specific proteins. These, they showed, can be used for research, for matching existing treatments to patients, and in the future – to initiate the kind of immune response one would want in a vaccine.
“Patients who suffer from pleural effusions secondary to cancer are in late stages of the disease,” Dr. Peled explains. “In those stages, the tumor is inoperable due to metastasis. This is the reason there’s no sufficient tissue to analyze. We hope that our ability to obtain tumor peptides from pleural effusions can lead to novel avenues of treatment for late-stage cancer.”
This is the first time that pleural effusions are put to the scientific use of identifying their repertoires of HLA-bound peptides. The same method used by Khazan-Kost and her colleagues, who focused on cancer, could also be used to diagnose and study other diseases that produce such effusions.
This study was supported by the Israel Science Foundation (ISF) and the Israel Cancer Association.