To Foam or not to Foam
We are all familiar with the phenomenon of foam associated with waves at sea. The waves break and insert air into the water, and the bubbles that are formed rise to the surface and create foam. If, however, we observe a stormy lake or a wave pool, we will not see much of a foam – certainly not as much as we see at sea. This difference between seawater and fresh water was reported in the professional literature already in 1929.
Foam consists of thin films of an aqueous solution surrounding air bubbles. In order for the foam to be stable, there must be a repulsive force between the two opposite sides of the films, otherwise they will become too thin to survive. This repulsive force is based on electrical charges (electrostatic repulsion), and is usually neutralized by the addition of salt. Therefore, it may have been expected that foam will not form in seawater.
Why this is of interest, you may wonder. Well, foam affects not only swimmers and surfers, but also the introduction of air into the water (vital for life at sea), and mainly cloud formation. Clouds consist of small water drops that result from condensation of water vapor in the air. The stable existence of clouds is contingent upon the presence of a small amount of salt in the drops. The salt particles originate from the bursting of the seawater foam bubbles, which releases them into the atmosphere.
This foam puzzle has been studied for decades, mainly in labs that study the physical chemistry of thin liquid films, and in bubble columns used in chemical engineering. Recently this longstanding mystery has been solved at Technion, as part of Yael Katsir’s doctoral work, under the guidance of Prof. Abraham Marmur from the Faculty of Chemical Engineering. A simple experimental model developed by the researchers, along with a theoretical model explaining the results, enabled the tracking of a single bubble formed below the surface of a salt solution and its rate of coalescence with the surface. To their surprise, the results of the experiment were completely different from the results of bubble column experiments. This contradiction eventually led to the solution. Contrary to the initial assumption that salt neutralizes the electrostatic repulsion, it turns out that in the present case some salt types actually create the repulsion and therefore the foam remains stable over time. The Technion researchers have demonstrated that the necessary conditions for this are high density of bubbles and relatively rapid movement toward each other.
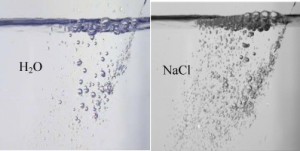 But, how do we know that what happens in a bubble column is the same as in se Waves? Another simple experimental system which simulates ocean waves breaking, consisted of an inclined syringe with a needle, from which a salt solution was jetted into a bath containing the same solution. High-speed photography of the process, done with the help of Gal Goldstein, enabled the researchers to monitor the development of the phenomena in space (on the vertical axis) and in time (on the horizontal axis). The attached photos clearly show the difference between the behavior of bubbles in salt water and fresh water: on the right, in salt water, high density of small bubbles and thick foam over the solution; on the left, in fresh water, much larger bubbles and sparse foam. These experimental results show that the data accumulated over the years with bubble column is indeed relevant to the formation of foam that occurs at sea.
But, how do we know that what happens in a bubble column is the same as in se Waves? Another simple experimental system which simulates ocean waves breaking, consisted of an inclined syringe with a needle, from which a salt solution was jetted into a bath containing the same solution. High-speed photography of the process, done with the help of Gal Goldstein, enabled the researchers to monitor the development of the phenomena in space (on the vertical axis) and in time (on the horizontal axis). The attached photos clearly show the difference between the behavior of bubbles in salt water and fresh water: on the right, in salt water, high density of small bubbles and thick foam over the solution; on the left, in fresh water, much larger bubbles and sparse foam. These experimental results show that the data accumulated over the years with bubble column is indeed relevant to the formation of foam that occurs at sea.
http://www.sciencedirect.com/science/article/pii/S2215038215300145




