The ‘Magic Bullet’ of Chemotherapy
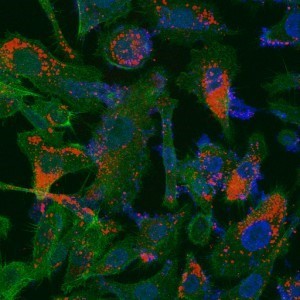
“Nano-skeletons’ (in red) delivered to human tissue infected by prostate cancer. The infected cells are colored in blue (PIP) and green (cytoplasmic); it is possible to see how the ‘nano-skeletons’ reach them
Florida native Dr. Beth Schoen, is part of a team developing a novel platform for delivering anti-cancerous drugs directly to its mark as part of her postdoctoral research at the Technion
Beth Schoen, born in Hollywood Florida, came to the Technion to conduct her postdoctoral research at age 26. In her very limited spare time she plays soccer for the leading all women’s soccer team – Maccabi Hadera – and studies Hebrew. “The Hebrew thing is no simple matter,” she confesses, “but I’m willing to make the effort, because it’s clear to me that Israel is where I want to live.”
Dr. Beth Schoen completed her undergraduate degree at the University of Florida, and her doctorate at Michigan State University in chemical engineering. “My doctoral studies focused on synthetic organic chemistry, particularly on the development of polymers with unique thermodynamic attributes especially resistant to high temperatures. These types of materials are used in part for the production of jet engine parts, body armor and Nomex (used for making fire-resistant gloves and overalls). One of our tasks was to create soft sheets that were not brittle, to be worn to be both bulletproof and fire resistant. It was a theoretical study, but as part of the process I also produced some of these polymers and tested them.”
Dr. Schoen planned to come to the Technion as part of her doctoral studies, but, she adds, “It didn’t work out, so I started to check where I could best fit in here in my future studies.” She decided to join Prof. Marcelle Machluf’s laboratory, at the Faculty of Biotechnology and Food Engineering, “I was eager to move from chemistry to biology and pursue cancer research in particular. I was very glad for the tremendous opportunity that Marcelle gave me in taking me on – perhaps it was because of my experience in nanomaterials and polymers.”
Prof. Marcelle Machluf’s research team consists of 17 female and 3 males students, researchers and technicians working on two main projects: (1) the development of scaffolds to rehabilitate damaged heart-tissue, and (2) the development of new technology to deliver drug treatment to damaged (sick) tissue (specifically related to cancer therapy). In an interview with her she focused on the second project.
“The current treatment for cancer involves radiotherapy and chemotherapy usually administered through intravenous infusion. The cancer drugs available are extremely effective, yet the way they are put to use in present day treatment, they also cause damage to healthy tissues. These are very potent drugs – they are intended to kill cancer cells – and on their way they also end up killing healthy ones.”
“The greatest damage is caused to rapidly dividing cells, which are similar to cancer cells. Hair follicle cells, for example, are a type of rapidly dividing cells and they damage easily from these types of treatment, which explains the hair loss in patients undergoing chemotherapy. Other side-effects include nausea and hearing loss, sometimes even leading to deafness. The drug Cisplatin for example, is a type of chemo drug used to treat various types of lung and breast cancers; some of its side-effects include damage to renal and immune system functioning, putting patients at risk to infections and diseases.”
These impediments are what fuel Prof. Machluf’s drive to develop a new drug delivery platforms capable of delivering anti-cancer drugs directly to the tumor without damaging healthy tissues on its way. “This is the top priority of cancer treatment: to develop a ‘magic bullet’ that target cancer cells,” explains Prof. Machluf. “And our new platform may be the solution to this great challenge.”
The new platform is based on ‘depleting’ specific cells – mesenchymal stem cells – so that there is nothing left of them save for the membrane. This membrane, called a ghost cells can be down sized to nano-vesicles, termed nano-ghosts, which can be loaded with any drug and delivered by injection directly into the blood stream. The immune system falls for the trap and does not recognize the ‘intruder,’ instead it treats these cells as if they were naturally part of the system and sends them to the afflicted area. On the way to their target they do not release the drug they are carrying and therefore do not do any damage to healthy tissues. Only upon reaching the malignant tissue, which they know how to identify, do they break down and secrete their contents at the site of the tumor cells.
This original idea was tested in a long series of experiments, and the results are very impressive: these nano-ghosts are in fact tumor selective, no matter the type of tumor. They ‘dash’ straight to the malignant tissue without emitting their drug on the way and without damaging healthy cells. Moreover, this unique ‘parcel’ increases the effectiveness of the treatment by ten-fold. Animal studies have shown that the employment of nano-ghosts for anti-cancer drug delivery have led to an 80% delay of prostate cancer – an unprecedented rate.
Still, there is a lot of work ahead, as Prof. Machluf’s research team works on improving the mechanism of this novel new platform: some of them are focusing on compatibility with specific drugs while others, like Dr. Beth Schoen, are concentrating on improving the nano-ghosts “This platform must be very precise,” explains Schoen. “It must be able to endure travelling through the entire human body, and release its contents only inside the tumor.”
The research is being carried out in collaboration with the Russell Berrie Nanotechnology Institute.


